Water Uptake and Transport in Vascular Plants

Why Do Plants Need So Much Water?
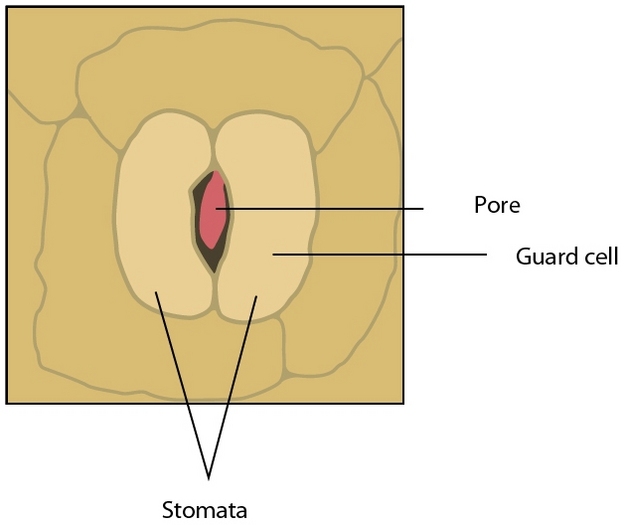
If water is so important to plant growth and survival, then why would plants waste so much of it? The answer to this question lies in another process vital to plants — photosynthesis. To make sugars, plants must absorb carbon dioxide (CO2) from the atmosphere through small pores in their leaves called stomata (Figure 1). However, when stomata open, water is lost to the atmosphere at a prolific rate relative to the small amount of CO2 absorbed; across plant species an average of 400 water molecules are lost for each CO2 molecule gained. The balance between transpiration and photosynthesis forms an essential compromise in the existence of plants; stomata must remain open to build sugars but risk dehydration in the process.
From the Soil into the Plant
Essentially all of the water used by land plants is absorbed from the soil by roots. A root system consists of a complex network of individual roots that vary in age along their length. Roots grow from their tips and initially produce thin and non-woody fine roots. Fine roots are the most permeable portion of a root system, and are thought to have the greatest ability to absorb water, particularly in herbaceous (i.e., non-woody) plants (McCully 1999). Fine roots can be covered by root hairs that significantly increase the absorptive surface area and improve contact between roots and the soil (Figure 2). Some plants also improve water uptake by establishing symbiotic relationships with mycorrhizal fungi, which functionally increase the total absorptive surface area of the root system.

Roots of woody plants form bark as they age, much like the trunks of large trees. While bark formation decreases the permeability of older roots they can still absorb considerable amounts of water (MacFall et al. 1990, Chung & Kramer 1975). This is important for trees and shrubs since woody roots can constitute ~99% of the root surface in some forests (Kramer & Bullock 1966).
Roots have the amazing ability to grow away from dry sites toward wetter patches in the soil — a phenomenon called hydrotropism. Positive hydrotropism occurs when cell elongation is inhibited on the humid side of a root, while elongation on the dry side is unaffected or slightly stimulated resulting in a curvature of the root and growth toward a moist patch (Takahashi 1994). The root cap is most likely the site of hydrosensing; while the exact mechanism of hydrotropism is not known, recent work with the plant model Arabidopsis has shed some light on the mechanism at the molecular level (see Eapen et al. 2005 for more details).
Roots of many woody species have the ability to grow extensively to explore large volumes of soil. Deep roots (>5 m) are found in most environments (Canadell et al. 1996, Schenk & Jackson 2002) allowing plants to access water from permanent water sources at substantial depth (Figure 3). Roots from the Shepard’s tree (Boscia albitrunca) have been found growing at depths 68 m in the central Kalahari, while those of other woody species can spread laterally up to 50 m on one side of the plant (Schenk & Jackson 2002). Surprisingly, most arid-land plants have very shallow root systems, and the deepest roots consistently occur in climates with strong seasonal precipitation (i.e., Mediterranean and monsoonal climates).

Flow = Δψ / R,
which is analogous to electron flow in an electrical circuit described by Ohm’s law equation:
i = V / R,
where R is the resistance, i is the current or flow of electrons, and V is the voltage. In the plant system, Vis equivalent to the water potential difference driving flow (Δψ) and i is equivalent to the flow of water through/across a plant segment. Using these plant equivalents, the Ohm’s law analogy can be used to quantify the hydraulic conductance (i.e., the inverse of hydraulic R) of individual segments (i.e., roots, stems, leaves) or the whole plant (from soil to atmosphere).
Upon absorption by the root, water first crosses the epidermis and then makes its way toward the center of the root crossing the cortex and endodermis before arriving at the xylem (Figure 4). Along the way, water travels in cell walls (apoplastic pathway) and/or through the inside of cells (cell to cell pathway, C-C) (Steudle 2001). At the endodermis, the apoplastic pathway is blocked by a gasket-like band of suberin — a waterproof substance that seals off the route of water in the apoplast forcing water to cross via the C-C pathway. Because water must cross cell membranes (e.g., in the cortex and at apoplastic barriers), transport efficiency of the C-C pathway is affected by the activity, density, and location of water-specific protein channels embedded in cell membranes (i.e., aquaporins). Much work over the last two decades has demonstrated how aquaporins alter root hydraulic resistance and respond to abiotic stress, but their exact role in bulk water transport is yet unresolved.
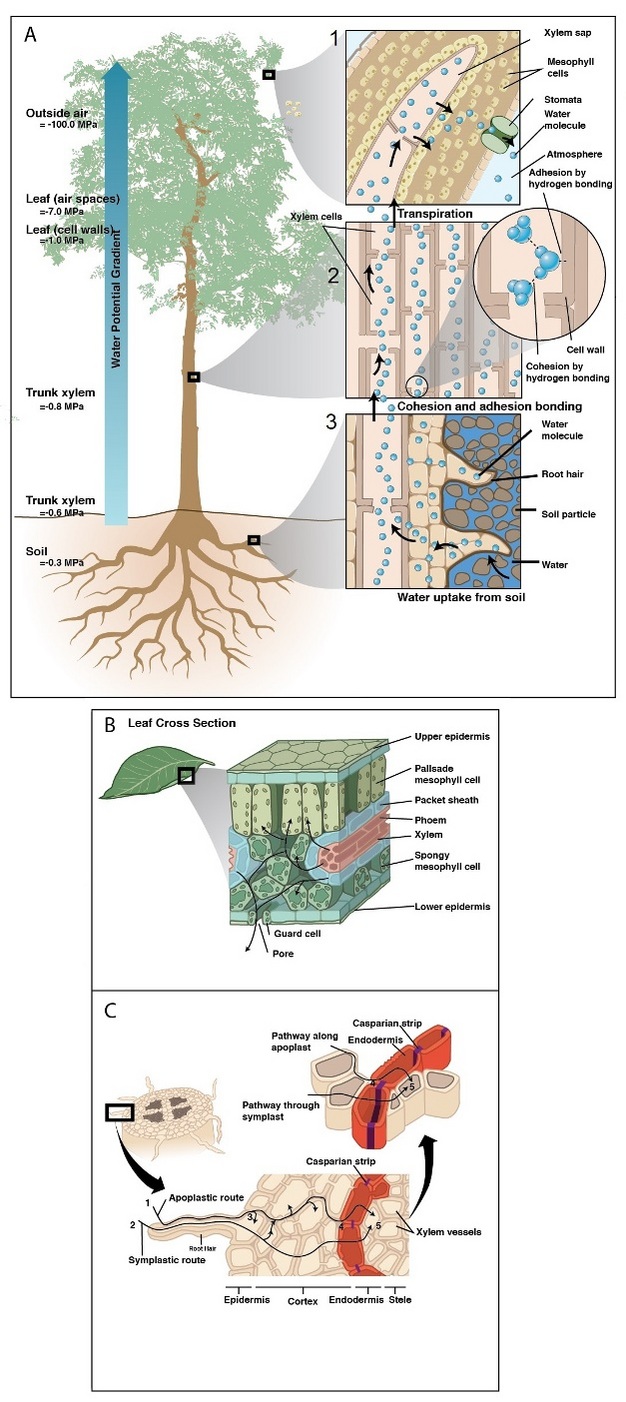
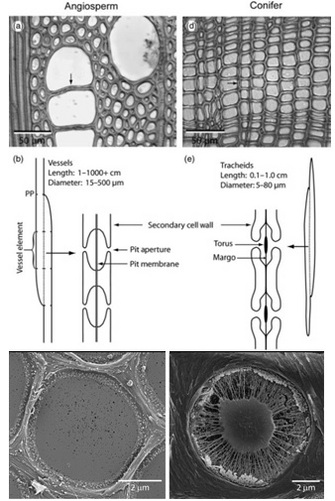
Once in the xylem tissue, water moves easily over long distances in these open tubes (Figure 5). There are two kinds of conducting elements (i.e., transport tubes) found in the xylem: 1) tracheids and 2) vessels (Figure 6). Tracheids are smaller than vessels in both diameter and length, and taper at each end. Vessels consist of individual cells, or “vessel elements”, stacked end-to-end to form continuous open tubes, which are also called xylem conduits. Vessels have diameters approximately that of a human hair and lengths typically measuring about 5 cm although some plant species contain vessels as long as 10 m. Xylem conduits begin as a series of living cells but as they mature the cells commit suicide (referred to as programmed cell death), undergoing an ordered deconstruction where they lose their cellular contents and form hollow tubes. Along with the water conducting tubes, xylem tissue contains fibers which provide structural support, and living metabolically-active parenchyma cells that are important for storage of carbohydrates, maintenance of flow within a conduit (see details about embolism repair below), and radial transport of water and solutes.
When water reaches the end of a conduit or passes laterally to an adjacent one, it must cross through pits in the conduit cell walls (Figure 6). Bordered pits are cavities in the thick secondary cell walls of both vessels and tracheids that are essential components in the water-transport system of higher plants. The pit membrane, consisting of a modified primary cell wall and middle lamella, lies at the center of each pit, and allows water to pass between xylem conduits while limiting the spread of air bubbles (i.e., embolism) and xylem-dwelling pathogens. Thus, pit membranes function as safety valves in the plant water transport system. Averaged across a wide range of species, pits account for >50% of total xylem hydraulic resistance. The structure of pits varies dramatically across species, with large differences evident in the amount of conduit wall area covered by pits, and in the porosity and thickness of pit membranes (Figure 6).
After traveling from the roots to stems through the xylem, water enters leaves via petiole (i.e., the leaf stalk) xylem that branches off from that in the stem. Petiole xylem leads into the mid-rib (the main thick vein in leaves), which then branch into progressively smaller veins that contain tracheids (Figure 7) and are embedded in the leaf mesophyll. In dicots, minor veins account for the vast majority of total vein length, and the bulk of transpired water is drawn out of minor veins (Sack & Holbrook 2006, Sack & Tyree 2005). Vein arrangement, density, and redundancy are important for distributing water evenly across a leaf, and may buffer the delivery system against damage (i.e., disease lesions, herbivory, air bubble spread). Once water leaves the xylem, it moves across the bundle sheath cells surrounding the veins. It is still unclear the exact path water follows once it passes out of the xylem through the bundle sheath cells and into the mesophyll cells, but is likely dominated by the apoplastic pathway during transpiration (Sack & Holbrook 2005).

Mechanism Driving Water Movement in Plants
Stephen Hales was the first to suggest that water flow in plants is governed by the C-T mechanism; in his 1727 book Hales states “for without perspiration the [water] must stagnate, notwithstanding the sap-vessels are so curiously adapted by their exceeding fineness, to raise [water] to great heights, in a reciprocal proportion to their very minute diameters.” More recently, an evaporative flow system based on negative pressure has been reproduced in the lab for the first time by a ‘synthetic tree’ (Wheeler & Stroock 2008).
When solute movement is restricted relative to the movement of water (i.e., across semipermeable cell membranes) water moves according to its chemical potential (i.e., the energy state of water) by osmosis — the diffusion of water. Osmosis plays a central role in the movement of water between cells and various compartments within plants. In the absence of transpiration, osmotic forces dominate the movement of water into roots. This manifests as root pressure and guttation — a process commonly seen in lawn grass, where water droplets form at leaf margins in the morning after conditions of low evaporation. Root pressure results when solutes accumulate to a greater concentration in root xylem than other root tissues. The resultant chemical potential gradient drives water influx across the root and into the xylem. No root pressure exists in rapidly transpiring plants, but it has been suggested that in some species root pressure can play a central role in the refilling of non-functional xylem conduits particularly after winter (see an alternative method of refilling described below).
Disruption of Water Movement
Water transport can be disrupted at many points along the SPAC resulting from both biotic and abiotic factors (Figure 8). Root pathogens (both bacteria and fungi) can destroy the absorptive surface area in the soil, and similarly foliar pathogens can eliminate evaporative leaf surfaces, alter stomatal function, or disrupt the integrity of the cuticle. Other organisms (i.e., insects and nematodes) can cause similar disruption of above and below ground plant parts involved in water transport. Biotic factors responsible for ceasing flow in xylem conduits include: pathogenic organisms and their by-products that plug conduits (Figure 8); plant-derived gels and gums produced in response to pathogen invasion; and tyloses, which are outgrowths produced by living plant cells surrounding a vessel to seal it off after wounding or pathogen invasion (Figure 8).
Abiotic factors can be equally disruptive to flow at various points along the water transport pathway. During drought, roots shrink and lose contact with water adhering to soil particles — a process that can also be beneficial by limiting water loss by roots to drying soils (i.e., water can flow in reverse and leak out of roots being pulled by drying soil). Under severe plant dehydration, some pine needle conduits can actually collapse as the xylem tensions increase (Figure 8).
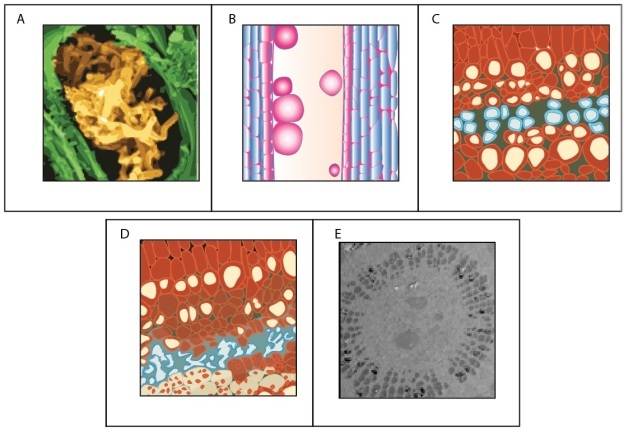
Water moving through plants is considered meta-stable because at a certain point the water column breaks when tension becomes excessive — a phenomenon referred to as cavitation. After cavitation occurs, a gas bubble (i.e., embolism) can form and fill the conduit, effectively blocking water movement. Both sub-zero temperatures and drought can cause embolisms. Freezing can induce embolism because air is forced out of solution when liquid water turns to ice. Drought also induces embolism because as plants become drier tension in the water column increases. There is a critical point where the tension exceeds the pressure required to pull air from an empty conduit to a filled conduit across a pit membrane — this aspiration is known as air seeding (Figure 9). An air seed creates a void in the water, and the tension causes the void to expand and break the continuous column. Air seeding thresholds are set by the maximum pore diameter found in the pit membranes of a given conduit.
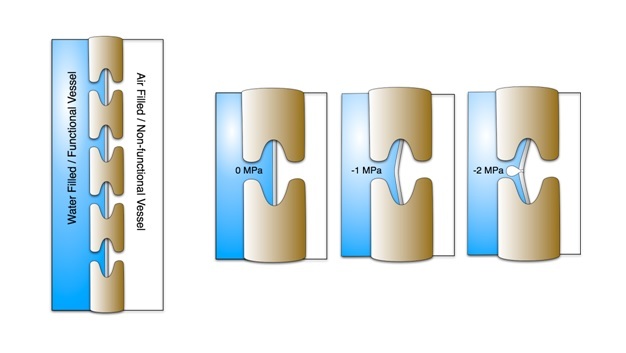
Fixing the Problem
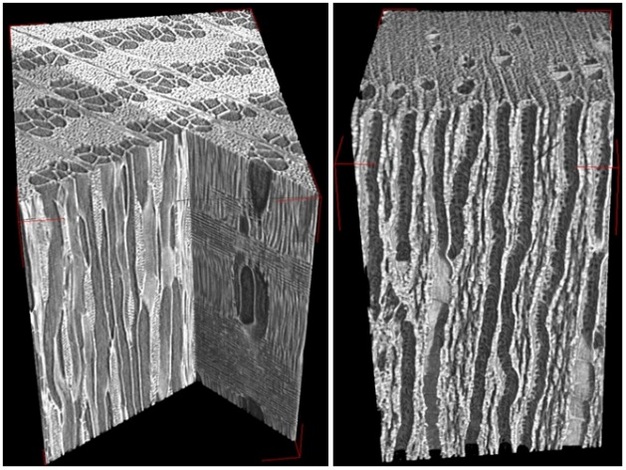
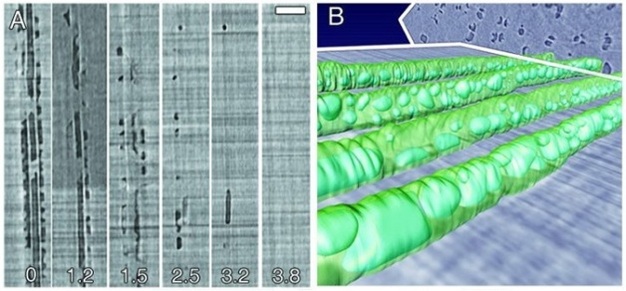
Failure to re-establish flow in embolized conduits reduces hydraulic capacity, limits photosynthesis, and results in plant death in extreme cases. Plants can cope with emboli by diverting water around blockages via pits connecting adjacent functional conduits, and by growing new xylem to replace lost hydraulic capacity. Some plants possess the ability to repair breaks in the water columns, but the details of this process in xylem under tension have remained unclear for decades. Brodersen et al. (2010) recently visualized and quantified the refilling process in live grapevines (Vitis vinifera L.) using high resolution x-ray computed tomography (a type of CAT scan) (Figure 10). Successful vessel refilling was dependent on water influx from living cells surrounding the xylem conduits, where individual water droplets expanded over time, filled vessels, and forced the dissolution of entrapped gas. The capacity of different plants to repair compromised xylem vessels and the mechanisms controlling these repairs are currently being investigated.
References and Recommended Reading
Agrios, G. N. Plant Pathology. New York, NY: Academic Press, 1997.
Beerling, D. J. & Franks, P. J. Plant science: The hidden cost of transpiration. Nature 464, 495-496 (2010).
Brodersen, C. R. et al. The dynamics of embolism repair in xylem: In vivo visualizations using high-resolution computed tomography Plant Physiology 154, 1088-1095 (2010).
Brodribb, T. J. & Holbrook, N. M. Water stress deforms tracheids peripheral to the leaf vein of a tropical conifer. Plant Physiology 137, 1139-1146 (2005)
Canadell, J. et al. Maximum rooting depth of vegetation types at the global scale. Oecologia 108, 583-595 (1996).
Choat, B., Cobb, A. R. & Jansen, S. Structure and function of bordered pits: New discoveries and impacts on whole-plant hydraulic function. New Phytologist 177, 608-626 (2008).
Chung, H. H. & Kramer, P. J. Absorption of water and “P through suberized and unsuberized roots of loblolly pine. Canadian Journal of Forest Research 5, 229-235 (1975).
Eapen, D. et al. Hydrotropism: Root growth responses to water. Trends in Plant Science 10, 44-50 (2005).
Hetherington, A. M. & Woodward, F. I. The role of stomata in sensing and driving environmental change. Nature 424, 901-908 (2003).
Holbrook, N. M. & Zwieniecki, M. A. Vascular Transport in Plants. San Diego, CA: Elsevier Academic Press, 2005.
Javot, H. & Maurel, C. The role of aquaporins in root water uptake. Annals of Botany 90, 1-13 (2002).
Kramer, P. J. & Boyer, J. S. Water Relations of Plants and Soils. New York, NY: Academic Press, 1995.
Kramer, P. J. & Bullock, H. C. Seasonal variations in the proportions of suberized and unsuberized roots of trees in relation to the absorption of water. American Journal of Botany 53, 200-204 (1966).
MacFall, J. S., Johnson, G. A. & Kramer, P. J. Observation of a water-depletion region surrounding loblolly pine roots by magnetic resonance imaging. Proceedings of the National Academy of Sciences of the United States of America 87, 1203-1207 (1990).
McCully, M. E. Roots in Soil: Unearthing the complexities of roots and their rhizospheres. Annual Review of Plant Physiology and Plant Molecular Biology 50, 695-718 (1999).
McDowell, N. G. et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytologist 178, 719-739 (2008).
Nardini, A., Lo Gullo, M. A. & Salleo, S. Refilling embolized xylem conduits: Is it a matter of phloem unloading? Plant Science 180, 604-611 (2011).
Pittermann, J. et al. Torus-margo pits help conifers compete with angiosperms. Science 310, 1924 (2005).
Sack, L. & Holbrook, N. M. Leaf hydraulics. Annual Review of Plant Biology 57, 361-381 (2006).
Sack, L. & Tyree, M. T. “Leaf hydraulics and its implications in plant structure and function,” in Vascular Transport in Plants, eds. N. M. Holbrook & M. A. Zwieniecki. (San Diego, CA: Elsevier Academic Press, 2005) 93-114.
Schenk, H. J. & Jackson, R. B. Rooting depths, lateral root spreads, and belowground/aboveground allometries of plants in water-limited environments. Journal of Ecology 90, 480-494 (2002).
Sperry, J. S. & Tyree, M. T. Mechanism of water-stress induced xylem embolism. Plant Physiology 88,581-587 (1988).
Steudle, E. The cohesion-tension mechanism and the acquisition of water by plants roots. Annual Review of Plant Physiological and Molecular Biology 52, 847-875 (2001).
Steudle, E. Transport of water in plants. Environmental Control in Biology 40, 29-37 (2002).
Takahashi, H. Hydrotropism and its interaction with gravitropism in roots. Plant Soil 165, 301-308 (1994).
Tyree, M. T. & Ewers, F. W. The hydraulic architecture of trees and other woody plants. New Phytologist119, 345-360 (1991).
Tyree, M. T. & Sperry, J. S. Vulnerability of xylem to cavitation and embolism. Annual Review of Plant Physiology and Molecular Biology 40, 19-38 (1989).
Tyree, M. T. & Zimmerman, M. H. Xylem Structure and the Ascent of Sap. 2nd ed. New York, NY: Springer-Verlag, 2002.
Tyree, M. T. & Ewers, F. The hydraulic architecture of trees and other woody plants. New Phytologist 119, 345-360 (1991).
Wheeler, T. D. & Stroock, A. D. The transpiration of water at negative pressures in a synthetic tree. Nature 455, 208-212 (2008).
Wullschleger, S. D., Meinzer, F. C. & Vertessy, R. A. A review of whole-plant water use studies in trees. Tree Physiology 18, 499-512 (1998).
Zimmerman, M. H. Xylem Structure and the Ascent of Sap. 1st ed. Berlin, Germany: Springer-Verlag, 1983.