What Is Soil?
Soil is a living, breathing, natural entity composed of solids, liquids, and gases.
Soil has five major functions:
- Provides a habitat for organisms
- Recycles waste products
- Filters water
- Serves as an engineering material
- Provides a medium for plumeria growth
Our focus will be on the fifth function. In this role, soil provides structural stability for plumeria and retains and relinquishes water and the nutrients necessary for plumeria growth.
An ideal soil for plant growth contains 50% porespace and 50% solids, with the porespace filled with equal parts air and water. This distribution rarely occurs because porespace varies with soil texture and soil management. For example, tilling increases porespace, while poor drainage and compaction reduce it.
Soil solids are a blend of mineral materials and organic matter. The mineral materials are typically weathered rock of varying sizes called sand, silt, and clay. The organic matter consists of decaying plant and microbial residues. The relative amounts of porespace and mineral and organic matter vary greatly among different soil types. But for plumeria growth, most soil scientists agree that 50% porespace, 45% mineral matter, and 5% organic matter make up an ideal ratio. The distribution of soils and porespace in compacted in poorly drained soil.
Even a small amount of organic matter can have a dramatic effect on the physical, chemical, and biological properties of soil.
|
1Brady, N. C. and R. R. Weil. 2004. Elements of the Nature and Properties of Soils, 2nd Edition. Atlanta, GA: Prentice Hall.
The Soil Profile
Most naturally occurring, undisturbed soils have three distinct layers of variable thicknesses. The layers are the topsoil, subsoil, and parent material. Each layer can have two or more sublayers called horizons. Collectively, the horizons make up the soil profile. The predominate parent material varies by location.
Soils’ properties vary with the soil depth. The surface soil, or topsoil layer (O and A horizon in Figure 1–2), usually contains less clay, but more organic matter and air, than the lower soil layers. Topsoil is usually more fertile than the other layers and has the greatest concentration of plant roots.
The subsurface layer (B and C horizon in Figure 1–2), known as subsoil, usually has a higher clay content and lower organic matter content than the topsoil.
Soil properties often limit the depth to which plant roots can penetrate. For example, roots will not grow through an impenetrable layer. That layer may be bedrock (Figure 1–3), compacted soil, or a chemical barrier, such as an acidic (very low) pH. A high water table can also restrict root growth due to poor soil aeration. Few big trees grow in shallow soils because big trees are unable to develop a root system strong enough to prevent them from toppling over. Shallow soils also tend to be more drought-prone because they hold less water and thus dry out faster than deeper soils. Water lost to runoff on shallow soils would instead be absorbed by a deeper soil. In addition, deep soils allow the roots to explore a greater volume, which means the roots can retain more water and plant nutrients.
Soils change in three dimensions. The first dimension is from the top to the bottom of the soil profile. The other two dimensions are north to south and east to west. The practical meaning of this three-dimensional variability is that as you move across a state, a county, or even a field, the soils change.
Five factors of soil formation account for this variation:
- Parent material
- Biological activity
- Climate
- Topography
- Time
Differences in even one of these factors will result in a different soil type. Soils forming from different parent materials differ. Soils forming from the same parent material in varying climates differ. Soils at the top of a hill differ from soils at the bottom. The top of the hill loses material due to natural erosion; the bottom gains the material from above. Considering the number of possible combinations of these five factors, it is not surprising that more than 450 unique soil series are currently mapped in North Carolina. Globally, more than 20,000 different soil series occur.


Physical Properties of Soil
The physical properties of soil are characteristics that can be seen, felt, or measured. These include color, texture, structure, and water-holding capacity. Such properties usually determine the suitability of soil as a growth medium. Some physical properties, such as texture, are not economically feasible to change on a large scale.
A soil’s fertility, which is a chemical property, is easier to change than the soil’s physical properties.
|
Color
Organic matter, the soil minerals present, and the drainage conditions all influence soil color. Color alone is not an indicator of soil quality, but color does provide clues about certain conditions. For example, light or pale colors in grainy topsoil are frequently associated with low organic matter content, high sand content, and excessive leaching. Dark soil colors may result from poor drainage or high organic matter content. Shades of red indicate a clay soil is well-aerated, while shades of gray indicate inadequate drainage (Figure 1–4). In well-drained soils of the NC mountains and piedmont, the subsoil colors are often shades of red, brown, and yellow. In poorly drained soils, the subsoil is grayer in color.
Texture
Soil texture, which refers to the proportions of sand, silt, and clay, influences nearly every aspect of soil use and management. Sand is the largest particle (at 2.0 to 0.05 mm), silt is much smaller (0.05 to 0.002 mm), and clay is the smallest (less than 0.002 mm) (Figure 1–5). To compare particle sizes, imagine that a sand particle is the size of a basketball. On that scale, a silt particle would be the size of a marble, and a particle of clay would be a pinpoint. How fine (clayey) or coarse (sandy) a soil is will determine many of the soil’s physical and chemical properties.
Much of a soil particle’s ability to react with water and nutrients is related to the amount of surface area available. When the individual particle size is small, more individual particles will fit in a given space, and thus make more surface area available. Clay, with its tiny particle size and platelike structure, holds water and nutrients effectively, while sand, which has a large chunky structure, does not. In addition to being smaller, clay particles are composed of different minerals than sand and silt, and a clay particle’s structure is more like a stack of paper plates than a grain of sand (Figure 1–6).
Table 1–1. Particle type, number of particles per gram, and the average surface area per gram.
| Particle Type | Diameter (mm) | Number of Particles per gram | Specific surface area (cm2/g) |
|---|
| Clay | < 0.002 | 90,260,853,000 | 8,000,000 |
| Coarse sand | 1.00-0.50 | 720 | 23 |
| Fine sand | 0.25-0.10 | 46,000 | 91 |
| Medium sand | 0.50-0.25 | 5,700 | 45 |
| Silt | 0.05-0.002 | 5,776,000 | 454 |
| Very coarse sand | 2.00-1.00 | 90 | 11 |
| Very fine sand | 0.10-0.05 | 722,000 | 227 |
Rocks and GravelRocks and gravel, which are large, coarse materials, can be found in many soils, but they are not considered when determining soil texture. Although some rocks and gravel in the soil will not affect plant nutrient uptake, they can make the soil difficult to dig. If the garden is mostly rocks or gravel, the soil will have a reduced water- and nutrient-holding capacity, and will be unfit for growing plants. In such a situation, it may be easiest to install raised beds and import soil.
|
The relative proportions of sand, silt, and clay determine a soil’s textural class (Figure 1–7). For example, a soil that is 12% sand, 55% clay, and 33% silt is in the clay textural class. Soil texture is a permanent feature, not easily changed by human activity. Consider a typical mineral soil that is 6 inches deep on 1 acre. That soil weighs about 2 million pounds. To change the sand content just 1% would require adding 20,000 pounds (or 10 tons) of sand. A 1% change in sand content would have minimal effect. A significant effect might require a 10% change, which would mean adding 100 tons of sand.
Adding organic matter is a more economically feasible alternative for improving soil. Adding organic matter does not change a soil’s texture—the percentage of sand, silt, and clay in the soil—but adding organic matter will alter soil structure by increasing the porespace and improving drainage. Gardeners can be successful with any soil texture, as long as they know the attributes and limitations of that soil.
Typically, laboratory procedures are used to determine the soil texture. It is possible, however, to use the procedure outlined in Figure 1–8 to determine the textural class by the “feel” method. It takes practice and calibration, but it can provide a reasonable estimate of the soil texture.
Sandy or Coarsely Textured Soils (Figure 1–9)
- Low in organic matter content and native fertility.
- Rapidly permeable and do not hold soil moisture.
- Nutrient leaching is a concern, so proper fertilization is a must. Apply smaller amounts of nutrients, and apply them more frequently.
- Low in cation exchange and buffer capacities.
- Well-suited for road foundations and building sites.
- Feel gritty.
Loamy or Medium-Textured Soils (Figure 1–10)
- Contains more organic matter.
- Permit slower movement of water and are better able to retain moisture and nutrients.
- Are generally more fertile.
- Have higher cation exchange and buffer capacities.
- Feel crumbly.
Clayey or Finely Textured Soils (Figure 1–11)
- Higher nutrient-holding capacity.
- Higher available water-holding capacity.
- Finely textured soils exhibit properties that are somewhat difficult to manage or overcome.
- Often too sticky when wet and too hard when dry to cultivate.
- May have shrink-and-swell characteristics that affect construction uses.
- Feel slippery.
How Do Soil Types Affect Gardeners?Compaction. Compaction occurs when pressure is applied to soil particles and the air and water are pushed out of the porespaces. Large, cubic sand particles are not easily compacted. Clay particles, small and platelike, are easily aligned and can compact, especially when wet. Compaction inhibits the movement of water, gases (air), and roots. Compacted soils have less infiltration, greater runoff, a higher risk of erosion, and more restricted root growth than soils without compaction. Water drains slowly, which may increase the likelihood of plant root diseases. Erosion. Sand particles are heavy, so they are not easily picked up and moved by water or wind. Clay particles are sticky, so they are not easily moved. Silty loam particles are light and not sticky, so erosive forces easily move them. Eroded soils are usually harder to till and have lower productivity than soils without erosion. The main causes of soil erosion in North Carolina are insufficient vegetative or mulch cover, and improper equipment and methods used to prepare and till the soil (Figure 1–12). Soil erosion can be minimized by following a few preventive measures:- Choose plants suited to the soil so they establish well.
- Mulch the surface each year with organic materials 1 inch to 3 inches deep.
- Adequately fertilize to promote vigorous, but not excessive, plant growth.
- Create a water diversion, such as a grass waterway, to capture and slow water movement.
- Align rows to follow the land’s contour so that water flowing downhill is slowed.
- Use proper tillage methods, such as not tilling when the soil is overly wet and not overtilling.
- Plant a winter cover crop.
- Consider installing rain gardens to capture sediment and runoff.
Surface Area. The most active part of a soil particle is its surface area. A particle’s surface is where nutrient exchange takes place. Sand particles have a small surface area relative to their mass, meaning they do not hold onto nutrients well. Clay particles have a large surface area relative to their mass, so a small amount of clay can add a significant amount of surface area to a soil, increasing the nutrient-holding capacity.
|
Structure
Soil structure refers to the grouping of individual soil particles into larger pieces called peds or aggregates. The structure of topsoil is usually granular and resembles chocolate cookie crumbs (Figure 1–13). Good granular structure allows rapid movement of air and water within the soil. Poor granular structure decreases movement of air and water. Good soil structure allows for extensive root development; poor structure can limit root growth. Supplying an adequate amount of organic matter and working the soil only when it is not excessively wet promotes good topsoil structure.
Water-Holding Capacity
Water enters the soil from precipitation or irrigation. It exits by draining from the soil, evaporating from the surface, and through transpiration from plant leaves. Water-holding capacity—the retention of water moving through soil—depends on differences in soil porespace. Ideal soils are half porespace with equal amounts of air and water filling the pores. Too much air means plants will wilt. Too much water means reduced plant vigor and susceptibility to root rot, which occurs due to anaerobic conditions.
Soils differ in the number of large (macro), medium (meso), and small (micro) pores. Macropores, which are more common in sandy soils, take up water more quickly and drain faster than meso- and micropores. This rapid draining from macropores is called “gravitational water” because the weaker forces of adhesion and cohesion in macropores cannot overcome gravity’s pull. Within 24 hours after a saturating rain, gravitational water reaches the lower soil horizons, and the soil is at field capacity: the meso- and micropores are still full of water because their adhesive and cohesive forces are stronger than gravity. Water in the mesopores is available to plants. But when the mesopores lose water as the soil dries through plant uptake and transpiration, soil moisture reaches the permanent wilting point. At the permanent wilting point, micropores are still full of water, but this water is so tightly held that it is not plant-available. Note that plants may wilt before the permanent wilting point if the plant transpires water through the leaves faster than it can take water up from the soil through its roots. This is why plants may wilt on hot days and then recover once the sun goes down and why plants can balance uptake with transpiration (Figure 1–14).
How to Remediate Compaction
Compaction is a likely problem if there has been recent construction or other traffic over the area. Deep cultivation, which is mixing the top 6 inches to 2 feet of soil with a tiller, disk, or hand tools, may be needed to loosen the soil. Incorporation of organic matter during deep cultivation can help to rehabilitate soil structure by creating aggregates and both macropores (for drainage) and mesopores (for plant-available water). Digging or cultivating soil when it is wet or excessively dry can destroy structure.
Be wary of quick fixes, such as starting over with a truckload of topsoil. Unfortunately, there are no standards on material sold as “topsoil.” New problems may be brought on site, such as weed seeds and disease organisms. Adding new topsoil to existing soil may also create drainage problems when water moves through the purchased topsoil and reaches the compacted layer. The water can pool and create unfavorable conditions for root growth.
Clay soils, which tend to hold excessive amounts of water and become compacted easily, present some tricky problems. Common mistakes are adding sand or peat moss to improve drainage. Adding sand to clay will reduce soil structure, lowering porespace. Adding peat moss will increase the clay soil’s high moisture-holding capacity. The best advice is to add smaller amounts of organic matter consistently every year, minimize compaction, and let soil biology naturally improve the structure over time.
Organic Matter
Organic matter consists of the remains of plants and animals and gives soil a gray to very-dark-brown color. Organic matter is home to many soil organisms.
Earthworms, insects, bacteria, fungi, and animals use organic matter as food, breaking it down to obtain energy and essential nutrients. Humus is the portion of organic matter that remains after most decomposition has taken place (Figure 1–16).
When organic matter decomposes in the soil, carbon dioxide is released and replaces some of the oxygen in soil pores. Carbon dioxide is dissolved by water in soil to form a weak acid. This solution reacts with soil minerals to release nutrients that can be taken up by plants. The digested and decomposing organic matter also helps develop good air-water relationships. In sandy soil, organic material occupies some of the space between the sand grains. This binds them together and increases water-holding capacity. In a finely textured or clay soil, organic material creates aggregates of soil particles. This allows water to move more rapidly around soil particles.
The amount of organic matter in the soil depends primarily on rainfall, air temperature, the kinds of plants that have been growing in a soil, management practices, soil temperature, and drainage. Soils that are tilled frequently are usually low in organic matter because tilling decreases residue particle size and increases the amount of air in the soil, increasing the rate of organic matter decomposition. Poorly drained soils tend to have a high percentage of organic matter because low oxygen levels limit decomposition organisms. To build organic matter in garden soil, till in compost when the garden is first created, but do not till in subsequent years. Instead, apply thin layers (1 inch to 3 inches), of organic mulch or compost to the soil surface each year (Figure 1–17). This material will break down, and the organic matter levels in the soil will gradually increase.
Improving the Soil
Good aeration and drainage, as well as the ability to hold adequate moisture and nutrients, are key components of an ideal soil environment. Although there is no cookbook recipe for creating this ideal environment, these are some of the most important strategies for improving soil quality:
- Minimize soil compaction (do not walk on garden beds or work wet soil) (Figure 1–18).
- Reduce drainage problems.
- Decrease erosion.
- Plant a cover crop (Figure 1–19).
- Incorporate organic matter.
- Provide a 1- to 3-inch layer of organic mulch on the soil’s surface.
Organic amendments can improve soils that suffer from high compaction, poor drainage, and erosion. Materials such as compost, manures, and pine bark are more effective and economical than vermiculite, peat moss, sand, topsoil, or perlite. Table 1–2 reviews the amounts of organic material to be added to soil per 100 square feet. When working in small areas, a general rule of thumb is to incorporate a 3- to 6-inch layer of organic material into the soil. The organic matter must be decomposed before plants can use the nutrients. The rate of decomposition of organic matter by soil organisms is affected by moisture, temperature, particle size, the carbon-to-nitrogen ratio, and nitrogen availability. The proper balance of carbon and nitrogen is needed for rapid decomposition, as are warm temperatures and adequate moisture. When using straw, leaves, or sawdust (which are high in carbon), add nitrogen fertilizer while the material is decomposing. Soil microbes use nitrogen during decomposition and may deprive plants, resulting in slow or stunted plant growth. Incorporating organic matter some months before planting the garden allows the material time to decompose and have plant-available nutrients in place for good plant growth.
Table 1–2. Organic Materials and Their Application Rates
| Organic Material | Amount to Be Added per 100 Square Feet |
|---|
| Compost | 10–20 cubic feet |
| Corncobs | 50 pounds (2 bushels) |
| Hay | 60 pounds (1 bale) |
| Leaves | 75 pounds (3–4 bushels) |
| Sawdust | 50 pounds (2 bushels) |
| Straw | 60 pounds (1 bale) |
| Wood chips | 50 pounds (2 bushels) |
Incorporating Soil Amendments
Conditioning soil requires increasing organic matter content to 25% by volume. Incorporating a minimum of 2 inches of material into the top 6 inches of soil will create approximately 8 inches of amended soil. These additions raise the planting bed, improving drainage and making plants more visible. Incorporating more than 50% organic matter may negatively affect plant growth. Be careful when using organic material, making certain that it is fully composted and not merely aged. Microbes attracted to partially decomposed materials will compete with plants for nutrients, especially nitrogen and sulfur, resulting in nutrient deficiencies and poor plant growth.
The best organic matter amendments for clay soils are pine bark (less than 1/2 inch in diameter) and composted leaf mold. The following amendments are not recommended because they do not adequately improve the physical properties of clay soil: peat moss, sand, hardwood bark, wood chips, and pine straw.
For sandy soils, organic matter amendments, such as pine bark or compost, will improve water retention.
Figure 1–19. A cover crop of white rye grass was planted in this annual flower bed. It is being turned under to add nutrients to the soil before planting.
Chemical Properties of Soil
There are strong relationships between soil physical properties and soil chemical properties. For example, surface area is directly related to chemical reactivity.
Cation Exchange Capacity (CEC)
The negative ends of two magnets repel each other. The negative end of one magnet attracts the positive end of another magnet. This same principle affects the retention of plant nutrients in soil. Some plant nutrients are cations, which have a positive charge, and some are anions, which have a negative charge. Just like the opposite poles on magnets, cations will be attracted to anions.
Soil particles are similar to a magnet, attracting and retaining oppositely charged ions and holding them against the downward movement of water through the soil profile. The nutrients held by the soil in this manner are called “exchangeable cations” and can be displaced or exchanged only by other cations that take their place. Thus, the negative charge on a soil is called the cation exchange capacity (CEC). Soils with high CEC not only hold more nutrients, they are better able to buffer or avoid rapid changes in the soil solution levels of these nutrients. A soil test will tell you the CEC number of your soil. Soils high in clay, silt, or organic matter will have a CEC number of 10 or greater, and no remediation is needed. Sandy soils will have a CEC number between 1 and 5. Adding organic matter to these soils will help increase the CEC.
Too Much of a Good Thing: Nitrogen Leaching
Soil testing provides valuable information on pH and plant-available nutrients. Test your soil before planting and every two to three years thereafter. Inexpensive soil test kits are unreliable. To accurately determine your soil characteristics and the proper amount of lime and fertilizer to apply, contact the NC Department of Agriculture and Consumer Services (NCDA&CS). The accuracy of these reports, however, depends on the quality of the sample submitted.
Soil Testing Just like magnets, negative charges repel negative charges. Soils with high CEC tend not to hold anions. As a result, water moving through the soil profile will leach negatively charged nutrients, such as chloride, nitrate, and sulfate out of the root zone. This leaching can result in contamination of groundwater, streams, and lakes or have other environmental implications (Figure 1–20). Excess fertilizer becomes a contaminant and can have adverse effects on human health. The U.S. Environmental Protection Agency has set standards for nutrients in groundwater used for drinking water. This is one of many reasons that appropriate levels of fertilization are essential.
Tips for Collecting a Good Soil Sample
- Collect samples with stainless steel or chrome-plated tools. Using brass, bronze, or galvanized materials could contaminate the sample.
- The bucket in which material is collected should be made of plastic.
- Make sure the collection bucket is clean because even small amounts of residual lime or fertilizer can affect the test results.
- Avoid taking samples from areas that are obviously different from the norm, such as wet spots, compost piles, animal urine spots, and brush piles, or from under eaves or sites where trash has been burned.
- Remove large pieces of organic material, such as roots, stalks, and leaves, from the sample.
- For gardens, new lawns, and other cultivated areas, sample to the depth the soil has been, or will be, tilled. For established lawns, collect the sample 2 to 4 inches deep. For trees and shrubs, take a sample to a depth of 6 inches near the plant’s drip line. Even if the soil looks the same, take separate samples for flower beds, vegetable gardens, fruit orchards, shrub borders, and lawn areas.
- If using a trowel or spade, dig a hole, then take a slice of soil down one side. Repeat this procedure in five to eight spots for each area to be tested. Mix these cores together to obtain one composite sample. If the soil is very wet, it could be more difficult to mix, but do not attempt to heat the soil to dry it (Figure 1–21).
- Place about a pint of the composite sample for each area sampled in a soil testing box and label with a return address on the side of the box. Make up a code that will be easy to remember, such as “flawn” for front lawn, “byard” for back yard, or “veg” for vegetable garden. Any combination of letters and numbers can be used. Make notes about where the samples came from so that when you receive the results, you can easily Identify how to treat the areas differently based on the results.
- Do not tape the boxes in any way. The lids are removed before the boxes go in the soil lab ovens, and tape makes this process difficult. Do not put the soil in a plastic bag before placing it in the box as doing so will prevent proper drying in the lab oven.
Fill out the soil test report sheet, giving as much information as possible. The required items are name, address, county, sample codes, and the crops planned. Reports are sent by mail only if there is a special request submitted to the lab. Otherwise, provide an email address on the form to receive notification that the report is complete and online. Farmers also use the form, so some of the information requested may not apply to gardeners (pounds of lime per acre, for example). Forms and boxes are available from the NCDA & CS or any county Cooperative Extension center.
Learn more about collecting soil samples in SoilFacts: Careful Soil Sampling—The Key to Reliable Soil Test Information (NC Cooperative Extension publication number AG-439-30). For detailed information about the soil test results, refer to NCDA&CS Agronomic Division’s Understanding the Soil Test Report.
How to Use a Soil Test Report
Fertilizing trees and shrubs in a landscape should be based on the amount of rainfall, soil type, the plant’s age, the amount of current growth, and desired future growth. Over application of fertilizer to home landscapes wastes money, contributes to pollution in our rivers, streams, lakes and estuaries, and may damage or kill desired plants. In addition, excess fertilizer can increase the likelihood of disease problems, lead to weak growth, attract pests, and increase the amount of pruning to keep mature plants within appropriate boundaries. A soil test report provides accurate guidance for applying fertilizer.
Example Soil Test Reports
Depending on the crop indicated when the soil sample was submitted, the soil test report provides results in one of two ways:
- Home garden scale: Recommendations for pounds of lime and a rate and grade of fertilizer per 1,000 square feet (for example, an area 50 feet by 20 feet or 10 feet by 100 feet).
- Farm/Forest scale: Recommendations for tons of lime and a rate and grade of fertilizer per acre.
At the home gardener scale:
- Measure the area to be limed or fertilized.
- Multiply the length by the width to determine the number of square feet.
- Divide by 1,000 to obtain the number of units to be treated.
- Multiply the number of units by the pounds of material to treat 1,000 square feet. This calculation will give the amount of fertilizer and lime needed (Figure 1–23).
Example 1:
If the area is 500 feet by 20 feet, and the suggested lime or fertilizer treatment is 30 pounds per 1,000 square feet:
- 500 × 20 = 10,000 square feet
- Divide 10,000 by 1,000 = 10 units
- Multiply 30 pounds times 10 units = 300 pounds of material (fertilizer or lime) per 10,000 square feet
Example 2:
If the area is 10 feet by 15 feet, and the suggested lime or fertilizer rate is 10 pounds per 1,000 square feet:
- 10 × 15 = 150 square feet
- Divide 150 by 1,000 = 0.15
- Multiply 10 pounds times 0.15 units = 1.5 pounds of material per 150 square feet
Or, look at fertilizer/lime calculations as ratios:
If 5 pounds of fertilizer are applied per 1,000 square feet, how many pounds should be applied to 150 square feet (using the garden size in Example 2)?< /p>
5 lb/1,000 sq ft = X lb/150 sq ft
5 lb × 150 sq ft /1,000 sq ft = X lb
750/1,000 = .75 lb
Example soil test reports and their recommended fertilizer applications can be found in Figure 1–24, Figure 1–25, Figure 1–26, and Figure 1–27.
Soil pH
Soil pH is a measure of the soil’s relative acidity or basicity. The pH scale ranges from 0 to 14. A pH of 7 is a neutral state, representing the value found in pure water. Values above 7.0 are basic, while values below 7.0 are acidic. The pH scale is logarithmic, meaning each unit has a 10-fold increase of acidity or basicity. Thus, compared to a pH of 7.0, a pH of 6.0 is ten times more acidic, and a pH of 5.0 is 100 times more acidic.
Nutrient Availability and pH
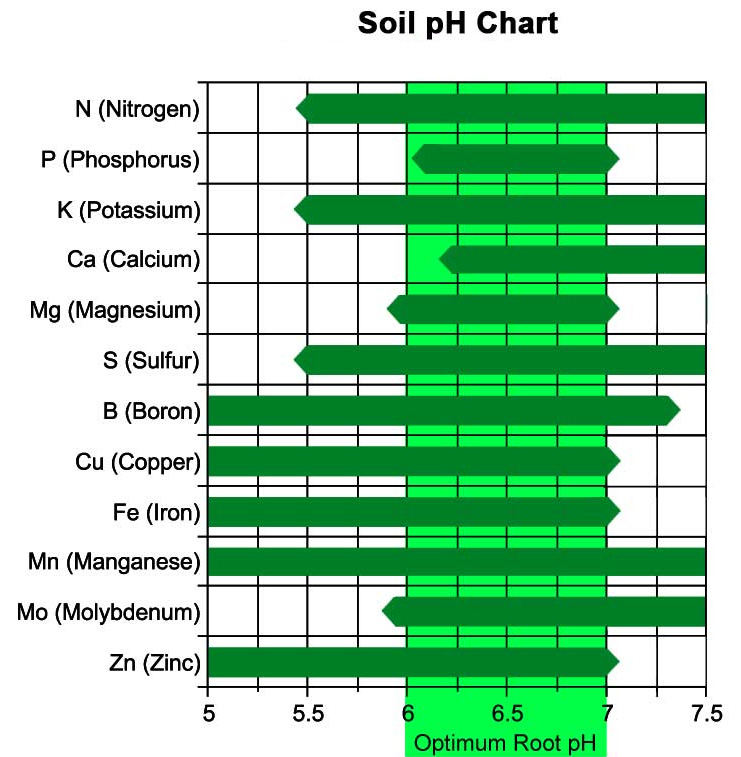
The optimum pH for a plant varies with organic matter content and plant type. Plant nutrient availability is strongly tied to the pH in the soil solution (Figure 1–28). Decreasing soil pH directly increases the solubility of the plant nutrients manganese (Mn), zinc (Zn), copper (Cu), and iron (Fe). Acidic soils make these nutrients more available. At pH values less than about 5.5, toxic levels of Mn, Zn, or aluminum (Al), a non-nutrient element very common in our southern soils, may be released. The impact of pH on nutrient availability is very important—both for maximum plant availability and to avoid potentially toxic levels at very low or very high pH.
The optimal pH for growth differs among plants. For example, regardless of organic matter content, azaleas and blueberries are well-suited for a soil pH of about 5.0. In contrast, asparagus can tolerate a basic soil with a pH up to 8.0. A soil pH of 6.5 to 7.0 is often considered “ideal” for most plants, but a little research can help you identify the proper pH for the plants you wish to grow. After obtaining a soil test report, you can take measures to adjust soil pH or select plants that will thrive at the current pH. Extreme pH measures of 4.0 (acidic) or 10.0 (basic) will support little plant life and are very difficult to modify.
Adjusting pH
If the soil pH is too basic for the desired plant, incorporating an acidic soil amendment such as pine bark or compost, or applying elemental sulfur, will lower soil pH. Apply sulfur with caution; too much can harm plants.
If the soil pH is too acidic, apply lime to raise the soil pH. There are two general classes of liming materials: calcitic (without magnesium) and dolomitic (with magnesium). Calcitic lime is composed of calcium carbonate (CaCO3) and can be used on soils high in magnesium. Dolomitic lime is a mixture of calcium and magnesium carbonates (CaCO3 and MgCO3), which is the preferred liming material for soils low in magnesium.
Knowing the soil type or even the current pH is not enough to determine the amount of lime needed. The texture of the soil, organic matter content, crop to be grown, target pH, level of soil acidity, CEC, type and amount of clay, and the current pH are all factors to consider in adjusting pH. Soils low in organic matter or high in sand content require less lime to change the pH than clay soils or those with high organic matter.
Lime is heavily regulated in North Carolina. Lime must be labeled with a guarantee of percent calcium and magnesium. The percent of calcium carbonate equivalent also must be included on the label, as well as the pounds of material that equal 1 ton of standard lime (Figure 1–29). Each type of lime must meet a screening requirement for particle size. Lime pellets are formed from lime that has been finely ground. The pelleted product is less dusty and easier to apply, but is slower to react with the soil.
Lime moves slowly in the soil and neutralizes acidity only in the area where it is applied. To be effective, it should be spread and thoroughly incorporated. It takes several months for lime to react in the soil, which is why it is good to soil test and plan for proper soil pH management. For established lawns, gardens, and ornamentals that require lime, apply the recommended amount up to 50 pounds of lime per 1,000 square feet in one application to the soil’s surface. For recommended rates over 50 pounds, wait several months to make a repeat application to avoid a surface buildup of lime. For new plantings where the area will be tilled, apply the entire recommended amount at one time.
Learn more in SoilFacts: Soil Acidity and Liming: Basic Information for Farmers and Gardeners.

Plant Nutrition and Fertilization
Many people confuse plant nutrition with fertilization. Plant nutrition refers to the needs of the plant and how a plant uses the basic chemical elements. Fertilization is the term used when these elements are supplied to the soil as amendments. Adding fertilizer during unfavorable growing conditions will not enhance plant growth and may actually harm or kill plants.
To complete their life cycle, plants need 17 essential nutrients, each in varying amounts (Table 1–3). Of these nutrients, three are found in air and water: carbon (C), hydrogen (H), and oxygen (O). Combined, C, H, and O account for about 94% of a plant’s weight. The other 6% of a plant’s weight includes the remaining 14 nutrients, all of which must come from the soil. Of these, nitrogen (N), phosphorus (P), and potassium (K), the primary macronutrients, are the most needed. Magnesium (Mg), calcium (Ca), and sulfur (S), the secondary macronutrients, are next in the amount needed. The eight other elements—boron, chlorine, copper, iron, manganese, molybdenum, nickel, and zinc—are called micronutrients because they are needed in much smaller amounts than the macronutrients.
Table 1–3. Relative Amounts (out of 100) of the Essential Nutrients Required by Most Plants
Primary Nutrients |
|---|
| Carbon (C) | 45 |
| Oxygen (O) | 45 |
| Hydrogen (H) | 6 |
| Nitrogen (N) | 1.5 |
| Potassium (K) | 1 |
| Phosphorus (P) | 0.2 |
Secondary Nutrients |
| Calcium (Ca) | 0.5 |
| Magnesium (Mg) | 0.2 |
| Sulfur (S) | 0.1 |
Micronutrients |
| Iron (Fe) | 0.01 |
| Chlorine (Cl) | 0.01 |
| Manganese (Mn) | 0.005 |
| Boron (B) | 0.002 |
| Zinc (Zn) | 0.002 |
| Copper (Cu) | 0.0006 |
| Molybdenum (Mo) | 0.00001 |
| Amounts unknown for Nickel (Ni) and Cobalt (Co) |
Soil Nutrients
For a plant to absorb an element, it must be in a chemical form used by the plant and dissolved in the soil water. In addition to those nutrients already dissolved in soil water, nutrients can be present in the soil in these forms:
- Undissolved or granular form, as from newly applied fertilizer
- Chemicals bound to soil particles
- The chemical structure of soil organic matter released by microbial decomposition
Undissolved or granular nutrients, and those that are chemically bound to soil particles, are not immediately useful, although they have the potential to benefit the plant. For many plant nutrients, the soil acts as a bank. Withdrawals are made from the soil solution, much as you would withdraw money from a checking account. The undissolved pool of soil nutrients is like a savings account. When checking funds are low, transfers are made from the savings account to the checking account. When a checking account is flush with money, some can be moved to savings for long-term retention. In the same way, for many plant nutrients, when the soil solution has excess nutrients, some bind to the soil to become temporarily unavailable, and some react with other chemical elements to form insoluble minerals, which can dissolve again later.
Several factors improve a plant’s ability to use nutrients:
- Type of soil: The more clay and organic matter a soil has, the higher its CEC will be, and the more cationic (positively charged) nutrients it will retain.
- Soil pH: The pH affects how tightly nutrients are bound to soil particles. If the soil pH is extremely high (basic) or very low (acidic), many nutrients become inaccessible to the plant because they are no longer dissolved in the soil water.
- Types of nutrients in the soil: Some nutrients affect the availability of other nutrients. In fact, an apparent deficiency of one nutrient may actually be caused by a large amount of another.
- Amount of soil water: Too much rain leaches nutrients from the soil. If there is too little water, the nutrients cannot dissolve and move into the plant.
- Anything that affects the plant’s growth: If growing conditions are good, a plant will absorb nutrients from the soil. If the plant experiences extremes in temperature, incorrect light levels, or waterlogged or compacted soil, it will have a limited ability to absorb nutrients. Also, plants in dormant stages absorb few nutrients.
The presence or absence of nutrients can cause outward symptoms to appear on the plant. Table 1–4 reviews the essential nutrients for plant growth and symptoms that may appear if a plant is suffering a deficency or an excess of that nutrient.
Fertilizers
Fertilizers provide some elements that might be lacking in the soil and stimulate healthy, vigorous growth. How much and when to apply fertilizers should be based on observing plant performance, a reliable soil test, and an understanding of the factors that affect growth: light, water, temperature, pests, and nutrition. Simply applying fertilizer because a plant is not growing adequately will not solve many plant problems (insects, disease, or poor drainage, for example), and, in fact, excess nitrogen can often increase insect and disease infestation.
All fertilizers are labeled with three numbers, giving the percentage (by weight) of nitrogen (N), phosphorus (P), and potassium (K). This is referred to as the fertilizer grade.
A 100-pound bag of fertilizer labeled 0-20-10 has 0 pounds of N, 20 pounds of P (reported as P2O5), 10 pounds of K (reported as K2O), and 70 pounds of filler. Filler is added to make the fertilizer easier to spread and to reduce the likelihood of burning plants with too much fertilizer (the fertilizer salts can pull water out of the plant). A fertilizer may also contain secondary macronutrients or micronutrients not listed on the label because the manufacturer does not want to guarantee their exact amounts.
Fertilizers can be divided into two broad categories: natural and synthetic.
Natural fertilizers are commonly misnamed “organic.” “Natural fertilizers” is a more accurate description because these materials can be both complex chemical substances containing carbon (organic materials) or inorganic ores, such as rock phosphate, which are mined. Natural fertilizers containing organic materials include manures and composts, animal byproducts (such as bone meal, blood meal, feather meal), and seed meals. Natural fertilizers that are inorganic ores include potassium and lime.
Natural fertilizers typically release nutrients at a slower rate and over a longer period than synthetic fertilizers because microorganisms are involved in a breakdown and release cycle called mineralization. Moisture, temperature, and the microbial species and populations in the soil affect mineralization. Some water-soluble natural fertilizers, such as fish emulsion, are available when rapid nutrient delivery is desired.
When using natural fertilizers, it is helpful to incorporate them and provide adequate moisture for active microbial populations. When packaged as fertilizers, natural fertilizers will have the nutrient analysis stated on the labels. How much to use varies with the nutrient content of the material. The age of the material is also a factor. Producers are not required by law to state the nutrient content on bulk organic materials, such as compost, manure, and sludges. The source of these materials should be investigated and possible analysis performed at the Plant, Waste, Solution, and Media Lab at the NCDA&CS Agronomic Division before applying large amounts to a home garden.
The age of the natural fertilizers is another important factor. When natural material decays and is rained on, it loses nutrients, especially potassium and, to some extent, nitrogen. Even natural sources of nutrients can be overappled and damage plants. Fresh manures, for example, may injure plants by adding excessive nitrogen or potassium, especially when applied in large quantities.
Natural fertilizers can be expensive if applied in amounts adequate to supply nutrients for good plant growth, but have the added benefit of improving soil structure and plant vigor. When applying natural fertilizers, calculate as closely as possible the amounts of nutrients being supplied. Always err on the low side of application rates, then test the soil and augment as recommended on the soil test report. The nutrient content may need to be supplemented with other natural or synthetic materials to achieve a balanced ration of nutrients.
Synthetic fertilizers are made through industrial processes or mined from deposits in the earth. They are purified, mixed, blended, and altered for easy handling and application. Most are noncarbonaceous chemicals from nonliving sources and are usually cheaper than natural fertilizers. In general, nutrients are more rapidly available to plants because they are more water-soluble or in a form plants can use. The disadvantage is that it may be easier to over apply a synthetic fertilizer than a natural one, which may result in fertilizer burn. In addition, synthetic fertilizers may not support beneficial microbial populations to the same extent as natural fertilizers.
Special-purpose fertilizers are packaged for plants such as camellias, rhododendrons, and azaleas (Figure 1–43). Some of the compounds used in these fertilizers have an acid reaction that can be beneficial to acid-loving plants if the soil they are growing in is naturally neutral or alkaline; however, most soils in North Carolina are usually acidic so these special fertilizers are unnecessary.
Fertilizer spikes or pellets are fertilizers compressed into a form placed in the soil or pots (Figure1–44). They are convenient, but are expensive per unit of fertilizer and do not provide uniform distribution. Nutrients are often concentrated around the spikes or pellets.
Liquid fertilizer can be purchased as a dry powder or as a concentrated liquid (Figure 1–45). Liquid fertilizers are frequently used for houseplants or as a starter solution for transplants. They tend to be more expensive per unit of fertilizer because they are made from refined chemicals.
Foliar fertilizers are dry powders or concentrated liquids that are mixed with water and sprayed on plants (Figure 1–46). Foliar feeding is used when insufficient fertilizer was applied before planting, when a quick growth response is wanted, when micronutrients are locked in the soil, or when the soil is too cold for the plant to use fertilizer in the soil. Foliar-applied nutrients are absorbed and used by the plant quite rapidly. They are expensive per unit of nutrient and only give short-term fertilization (completely absorbed within one to two days). Relying totally on foliar fertilization can be time consuming because the fertilizer must be applied regularly. Improper foliar application of fertilizers can also lead to plant tissue burn.
Learn more about fertilizer usage and nutrient concentrations in the North Carolina Agricultural Chemicals Manual, Chapter IV – Fertilizer Use.
Fertilizer TermsFertilizer analysis: The minimum amount of each element in a fertilizer as stated on the label, such as 16-4-8. Fertilizer ratio: The relative proportion of N, P2O5, and K2O. The ratios of 16-4-8 and 8-2-4 are both 4:1:2, which means 4 parts nitrogen to 1 part phosphorus to 2 parts potassium. Balanced fertilizer: A fertilizer containing equal parts of each major element, such as 10-10-10. Complete fertilizer: A fertilizer containing nitrogen, phosphorus, and potassium. Examples of commonly used fertilizers are 10-10-10, 16-4-8, and 12-4-8. Incomplete fertilizer: A fertilizer missing one or two of the macronutrients, such as 0-20-0. Weed and feed fertilizers: A combination of fertilizer and herbicide. They are often used on lawns to prevent certain weeds from germinating, or to kill existing broadleaf weeds. High analysis: A fertilizer containing 30% or more active nutrients, such as ammonium nitrate 33-0-0. The cost per bag is usually more, but the cost per pound of nutrient is less, lowering the cost for fertilizing a given area.
|
Incomplete fertilizers can be used separately or combined to supply the needed nutrients, often at a reduced cost compared to using a complete fertilizer. For example, gardeners who have a soil with sufficient P and K can save money by applying a nitrogen-only fertilizer, such as ammonium nitrate (34-0-0). If a soil test indicates N and K are needed, but not P, use an appropriate amount of ammonium nitrate and muriate of potash (0-0-60), a naturally occurring material composed almost entirely of potassium, processed to remove impurities and concentrate the product. If a soil needs only P, use triple super phosphate (0-46-0), or for an organic nutrient source apply bone meal (approximately 3-15-0; note that this will add some N) or compost.
Regardless of the fertilizers used, be aware that excess fertilizer can damage plants and move into our stormwater systems, which can cause serious environmental problems.
|
Plant Nutrients and the Environment
Fertilizer misuse causes environmental and water quality issues. Nitrogen fertilizers, for instance, break down into ammonium and nitrate. The nitrate form of N, while essential for plant growth, is highly mobile and can move through the soil after rainfall or irrigation and contaminate drinking water supplies. Phosphorus holds tightly to soil particles and does not leach through the soil, but affects water quality through runoff and soil erosion. Excess nitrogen and phosphorus are associated with algal blooms (heavy growth of aquatic plants) and limited oxygen, and cause fish kills in lakes, bays, and nonflowing water bodies.
There are several ways to reduce fertilizers’ impacts on water quality:
- Apply only materials that are recommended based on results of a soil test. If possible, use slow-release fertilizers and incorporate into the soil. Avoid applying excess nitrogen and phosphorus fertilizer.
- Calibrate fertilizer spreaders properly and clean spreaders over the lawn area instead of a hard surface.
- Keep the amount of hard surfaces in a landscape to a minimum. When installing a new sidewalk or patio, consider using gravel, porous concrete, stepping-stones, wood decking, or bricks on a sand base (Figure 1–47).
- Avoid applying fertilizer to hard surfaces, such as sidewalks, patios, driveways, and streets. Sweep up material that falls on hard surfaces.
- Maximize water absorption by aerating lawns and incorporating organic matter in planting beds and gardens.
- Prevent runoff by turning off irrigation when the soil is no longer absorbing water.
- Avoid applying fertilizer in natural drainage areas or ditches.
- Minimize soil erosion by using ground covers, windbreaks, terraces, and mulches.
- Mulch under trees and shrubs to reduce impact of falling water.
- Maintain a lawn border around planting areas and plant a grass strip between rows in fruit and vegetable gardens.
- Plant cover crops on bare soil, such as barren vegetable gardens.
- Use a rain barrel under drains to collect runoff and direct excess runoff from roofs onto grassy areas (Figure 1–48).
When to Apply Fertilizer
Soil type affects the frequency of fertilizer application. Sandy soils require more frequent applications of smaller amounts of nitrogen and potash than do clay soils because these nutrients leach more readily in sandy soils. Other factors that affect application frequency include the plant to be grown, the amount of plant growth desired, the amount of water, and the type and release rate of fertilizer applied.
The best time to apply fertilizer and the most effective method of applying it depend on the type of plants being grown. Leafy vegetables require more nitrogen than root crops. Corn is a heavy nitrogen feeder and may require several small nitrogen applications when actively growing. Most established woody plants perform well without fertilization, or with just one application per year. Young plants may benefit from several light applications of fertilizer per year.
Fertilizer is needed when plants are actively growing, never when they are dormant. Nitrogen application will have its greatest effect three to four weeks after application. Excess or improperly timed nitrogen can delay flowering and fruiting or promote tender new growth vulnerable to frost or freeze damage.
Research has shown it is best to broadcast or incorporate fertilizer uniformly over an area rather than concentrating fertilizer in holes or bands in the soil. The most effective method of fertilizing a large area is with a fertilizer spreader; for home gardens, hand fertilization works fine. For new plantings, incorporate fertilizer into the soil and mix it thoroughly. For established plantings, surface application is appropriate.
When fertilizing from overhead, make certain plant foliage is dry and use a broom to brush fertilizer off the foliage, or water thoroughly after applying fertilizer to remove it from plant leaves to prevent burn spots. It is not necessary to remove mulch when fertilizing; irrigation or rainfall will carry fertilizer to the roots. Fertilization should be reduced or delayed during dry weather because the salts in the fertilizer can burn roots if there is inadequate moisture.
Calibrating a Spreader Fertilizers are more effective if they are applied at the proper rate and with uniform coverage. To accomplish this, calibrate the spreader, which requires a little labor and math. The two types of spreaders used to apply fertilizer and lime are drop spreaders (Figure 1–49) and rotary spreaders (Figure 1–50). The amount of fertilizer that is spread depends on the opening setting, the type of fertilizer, and the speed at which the spreader is pushed. The drop spreader has a series of holes at the base that can be adjusted to apply different amounts of material. With the rotary spreader, the fertilizer falls into a rotating plate and is spread by the centrifugal force of the plate spinning. Instructions for calibrating a spreader should be available on the Internet at the home page for the spreader manufacturer.
|





 Roots need oxygen for growth, maintenance of the root system, and the uptake of water and nutrients. The provision of a good supply of air for fast growing plants is of vital importance for obtaining good results. A shortage of air in the early stages of cultivation leads to a poorly developed root system, which hampers growth resulting in a smaller yield.
Roots need oxygen for growth, maintenance of the root system, and the uptake of water and nutrients. The provision of a good supply of air for fast growing plants is of vital importance for obtaining good results. A shortage of air in the early stages of cultivation leads to a poorly developed root system, which hampers growth resulting in a smaller yield. Two different methods of harvesting can be used to exploit peat deposits: the cheapest but least efficient method consists of “shaving off” the top layer of peat. The disadvantage of this technique is that the structure is less coarse which has an undesirable effect on the air/ water relationship.
Two different methods of harvesting can be used to exploit peat deposits: the cheapest but least efficient method consists of “shaving off” the top layer of peat. The disadvantage of this technique is that the structure is less coarse which has an undesirable effect on the air/ water relationship.
 As mentioned, the root system will only increase in volume for as long as the rest of the plant continues to grow. However, transpiration from the leaves can also cause more roots to form in order to pump up the water needed. In the end, an equilibrium is established between the roots and the plant. A general rule of thumb is that the root system should comprise 30% of the total plant volume. Although this rule applies fairly consistently to plants in the open air, in substrate culture this does not always have to be the case. You can grow large plants in small pots as long as you supply them with water and nutrients and do not allow the pot to get too dry or too wet. To reduce the chance of this happening, we advise a large medium volume.
As mentioned, the root system will only increase in volume for as long as the rest of the plant continues to grow. However, transpiration from the leaves can also cause more roots to form in order to pump up the water needed. In the end, an equilibrium is established between the roots and the plant. A general rule of thumb is that the root system should comprise 30% of the total plant volume. Although this rule applies fairly consistently to plants in the open air, in substrate culture this does not always have to be the case. You can grow large plants in small pots as long as you supply them with water and nutrients and do not allow the pot to get too dry or too wet. To reduce the chance of this happening, we advise a large medium volume.

 Pythium infection on tomato roots in coco coir.
Pythium infection on tomato roots in coco coir.