The Role of Potassium (K)
Potassium fertilizer is the “K” in the N-P-K numbers listed on every bag of fertilizer. All fertilizers have three numbers on the bag, such as 11-11-13 or 10-10-10. The numbers represent the relative amounts of Nitrogen (N), Phosphorus (P), and Potassium (K) in the fertilizer. They are always listed in that order and are referred to as that fertilizer’s N-P-K value. Soils vary in the amount of naturally occurring potassium that is available to plants.
Potassium is a chemical element with the symbol K (derived from Neo-Latin, also know as kalium) and atomic number 19. It was first isolated from potash, the ashes of plants, from which its name derives. In the Periodic table, potassium is one of seven elements in column (group) 1 (alkali metals). All elements in Group 1 have a single valence electron in the outer electron shell, which is easily removed to create an ion with a positive charge – a cation, which combines with anions to form salts. Potassium in nature occurs only in ionic salts. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and reacts vigorously with water, generating sufficient heat to ignite the hydrogen emitted in the reaction and burning with a lilac-colored flame. It is found dissolved in seawater (which is 0.04% potassium by weight) and is part of many minerals.
Naturally occurring potassium is composed of three isotopes, of which 40 K is radioactive. Traces of 40 K are found in all potassium, and it is the most common radioisotope in the human body.
Potassium is chemically very similar to sodium, the previous element in Group 1 of the periodic table. They have similar ionization energy, which allows for each atom to give up its sole outer electron. That they are different elements that combine with the same anions to make similar salts was suspected in 1702 and was proven in 1807 using electrolysis.
Most industrial applications of potassium exploit the high solubility in water of potassium compounds, such as potassium soaps. Heavy crop production rapidly depletes the soil of potassium, and this depletion is prevented and remedied with agricultural fertilizers containing potassium, accounting for 95% of global potassium chemical production.
Potassium ions are necessary for the function of all living cells. The transfer of potassium ions through nerve cell membranes is necessary for normal nerve transmission; potassium depletion in excess can result in numerous abnormalities, including an abnormal heart rhythm and various electrocardiographic (ECG) abnormalities. Fresh fruits and vegetables are good dietary sources of potassium. The body responds to the influx of dietary potassium, which raises serum potassium levels, with a shift of potassium from outside to inside cells and an increase in potassium excretion by the kidney.
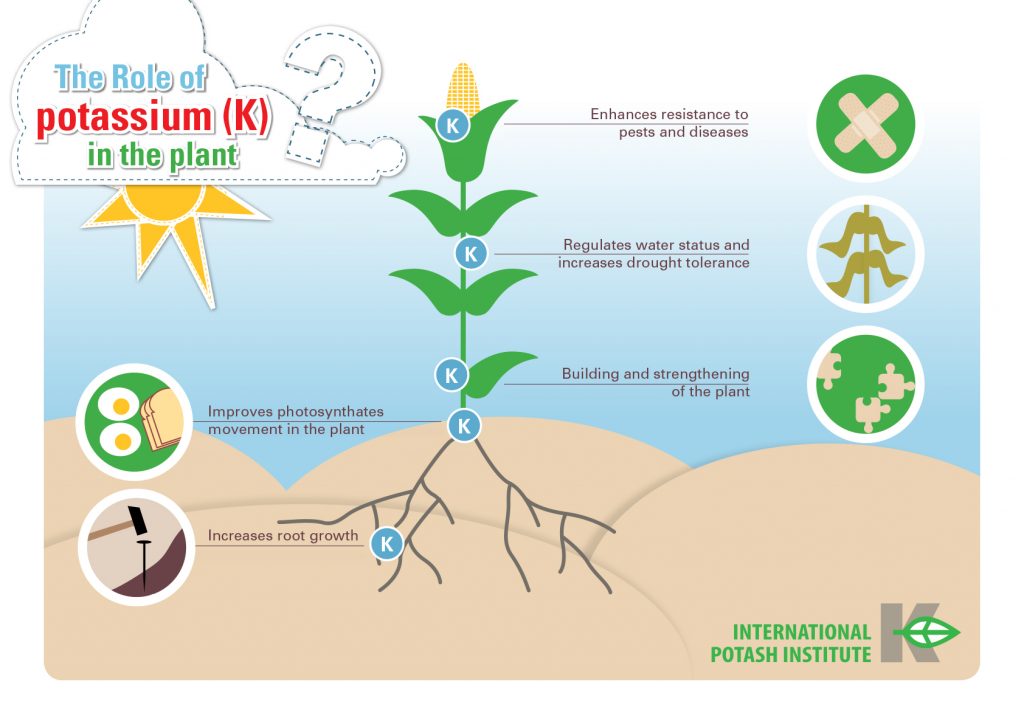
Plants need potassium for metabolic processes.
Photosynthesis, protein synthesis, and enzyme activation needed for plant growth and reproduction could not occur without potassium. Potassium affects many attributes of a plant, including taste, size, and shape.
Potassium is one of the most critical minerals needed by plants for a variety of physiological functions:
- disease resistance
- building proteins
- opening and closing their stomata (the pores that allow for gas exchange in photosynthesis)
- other required physiological functions
Signs of potassium deficiency in plants are leaf edges turning brown (looking as though they are getting scorched) and leaves turning yellow, especially at the tips, while the veins remain green.